“Aimovig.” A drug developed just for migraine disease, the very first preventative: “Aim.” “Vig” = Vigor, what we will have more of, even if improvement is only 10-20%. Generic name: erenumab. Drug company: Amgen / Novartis, who are not paying me.
Side effects: possible injection site irritation and constipation.
Have you looked at the side effects of sumatriptan lately? Or topiramate? nadalol? DHE? Botox?
The cost is 30% less than predicted. I contacted Amgen to find out details and haven’t heard back yet, but there is even a program called “Aimovig Ally” to help people pay for it.
Unlike any other drug, even those like the triptans developed specifically for migraine, the CGRP antagonists work BETTER over time, not worse. There is no sign of the rebound effect, which forces chronic sufferers to choose which days to treat because taking any meds available now more than twice per week will produce more migraine. Naproxen causes the least rebound, but it kills your stomach (it did mine and now I can no longer take NSAIDs at all). Preventatively, it’s been seen that even after cessation of treatment, the positive effects of CGRP antagonists continue. The acute pill that I’m trying now leaves my brain feeling cushioned for days. It can be taken daily with no overuse issues, but I haven’t needed to take it every day.
For someone who was chronic daily in 2014, before beginning my three clinical trials, this truly does feel like a miracle. It’s not a cure. Some people may not have tremendous improvement from Aimovig, but some might. Even a 20% difference for someone who is chronic has a huge impact.
I understand not wanting to hope (here is a poem I wrote for migraine.com about the hope I was feeling during the trials). I understand being burned over and over by new medications, whether it’s because they don’t work, or do work but have horrible side effects. I even had major surgery hoping to no longer be chronic afterward, and was initially WORSE, and then only marginally better: still chronic daily but fewer hospitalizations. I’m 44 and have been living with this disease for 40 years. There is nothing that would stop me from trying this drug, not cost (I would fight the insurance companies to the death), not potential side effects (there aren’t any), not fear of needles (I got over that fast when Imitrex auto-injectors came out), not wondering about long term effects. I want to live my life NOW. I want to feel better. I am so thrilled that the release of this medicine got sped up and that the cost went down. I am excited to the verge of tears for the entire migraine community. I just didn’t expect people to resist it so hard due to fear of long term dangers that wouldn’t yet be known.
Here is a link to a video of my last Teva injection on Migraine.com.
With the triptans, DHE, SSRIs, etc, there is danger of serotonin syndrome. Even cough syrup and sudafed can cause it. The release of CGRP in the brain causes migraine. The new drugs stop that release, either before the migraine (Aimovig) or after (the trial pill I have now). Unlike serotonin, CGRP is only associated with pain and migraine brains have more of it. Lack of the chemical does not cause problems the way lack of serotonin does. CGRP antagonists also don’t cause vasoconstriction, so there is no heart or blood pressure risk. For many many years migraine was thought to be caused by constricted blood vessels. Until scientists realized it wasn’t. Migraine is a neurological disease caused by a certain type of sensitive brain. It is more prevalent in women and runs in families. It’s not caused by stress, or wine, or chocolate, or making dumb choices, or being too ambitious or too “high maintenance.” It is a disease like epilepsy. Like Parkinson’s. And it needs to be maintained, with preventatives and abortives and rescue meds.
Until now, all three of those categories contained some dangerous medications, made for other illnesses (mostly) and lots of caveats. Many of them taken together cause serotonin syndrome. ALL of the abortives, even DHE which was long believed to not cause the issue and is still used to break up intractable migraine despite incredibly difficult side effects, cause rebound or overuse headache (I prefer the term rebound so as not to blame the patient). Aimovig is a preventative, but the abortive versions will not. cause. rebound. This is huge. It is a new, novel type of medication which has been able to be developed due to advances in medical science. The drugs have been in trials for years. My Amgen trial was in Phase 2 in 2014, meaning Phase 1 was long over. My Teva trial was Phase 3. The abortive medication I’m taking now was shelved years ago because it was found to cause liver issues. So the studies were stopped until they could determine why, and fix that. If Aimovig had caused anything similar, it too would have been put aside.
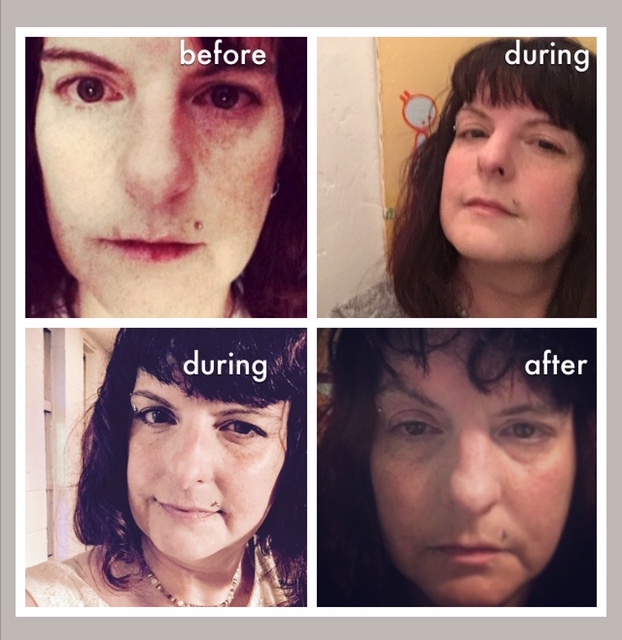
It’s not exactly fair for me to use the photos above, because sure, I had migraine while participating in the studies. During the Amgen open label phase, I was getting the lowest dose of what is now Aimovig. I was still going through a lot of medications and needing ER visits (read Kerrie Smyre’s interview with me here). But somehow things were just more manageable. During the Teva study, the doses varied because that’s what they were looking at: which doses worked better. Read here about how one month I had NO migraine at all. Obviously that was the right dose. After the second study was over, yeah, I suffered. Look at that swollen eye. But either that was from using the two different types in a row, or a coincidence. My last injection was in June; by October I was about as well as I had been during the five months between the two studies (better than before the first study by a lot). The fourth photo was taken in August.
Here are the facts of my life over the past five years. In early 2013 I had to resign my position with Parks & Rec and apply for Disability, which was approved right away. I was in bed most days, totally housebound, sick as a dog, all the time. In August, John began our Migraine 365 project, which I provided the writing for, but that is all I was doing. In 2014, my friend contacted me about a clinical trial she was running that had seen very positive results. She wanted me to participate. With no hesitation, I signed up. During the double blind part of the study, I felt improved enough to begin volunteering at the history museum. I took X to try out for a play and she did not get a part, but enthusiastically participated in backstage crew during tech week and got to be an extra, on stage after all. I was asked to join the theatre group’s advisory board. I said yes. I tried out for a play myself, with the whole family, and didn’t miss a single rehearsal. Throughout 2015, in the open label phase when I was for sure getting the med, I continued volunteering, spending less and less time in bed. In 2016 I began contributing to the development of a new online independent news journal. The Amgen trial ended. My friend found another trial for me. One of my favorite migraine bloggers interviewed me about the trials for Migraine.com. Health Union, the company which owns Migraine.com, then asked me to join them as a writer and patient advocate, so I began being paid to blog for a much larger audience. In 2017, I went to Philadelphia for a Health Union conference. The second study ended, I had a couple of bad months then improved again, and in March 2018 my friend found yet another study I could participate in. The drug is still a CGRP antagonist, but this time an abortive tablet to take after the pain starts. Right at the same time I rejoined the workforce and got a part time dream job at my local library. None of that would have been possible without the CGRP-antagonist clinical trials. I was given my life back, handed to me on a silver platter labeled “CGRP.”
I do understand that very long term effects, like 10 – 20 years into treatment, can’t possibly be known now. But if you’re in severe pain every single day, wouldn’t it be worth the risk? When its initial side effect profile is so low? I have been so excited for years, and waiting for this day. I was a willing guinea pig, but with “clinical trial guilt” because I was getting relief others in the community didn’t have access to. All I wanted was for everyone to have the same chance I was getting. And now that chance, in the form of a once-monthly self injection that costs 30% less than expected, is here. I hope that the Aimovig Ally program will help a lot of people and that insurance companies will wise up fast so that everyone suffering can get their lives back the way I did. And I guess if there are people I care about who don’t want to try it, I need to step back and accept that. Every person is different. There are some very real reasons, like other health problems so severe that improvement would be hard to determine; to them it would feel like just adding one more thing to a soup of a messed up situation, though I would still say removal of the CGRP chemical would help migraine and probably not affect anything else. I myself did experience significant temporary worsening (read that article here) after the end of the Teva trial, and hemiplegic migraine with lasting numbness and tingling. If new symptoms were somehow caused by the removal of the second study drug, I am the only one who had that happen out of the thousands and thousands in the trial. If it was caused by the study drug(s), it was because of using one followed by another with a much different mechanism, which no one would ever be doing post-FDA acceptance. And despite those disconcerting changes and the aneurysm scare (that came to naught), I still jumped on the opportunity to start yet another trial. But I’m just me, and I can’t expect everyone in the community to want to take a risk on an expensive new drug.
But you know what?
I’m a lot better. And I wouldn’t trade it for the world.

